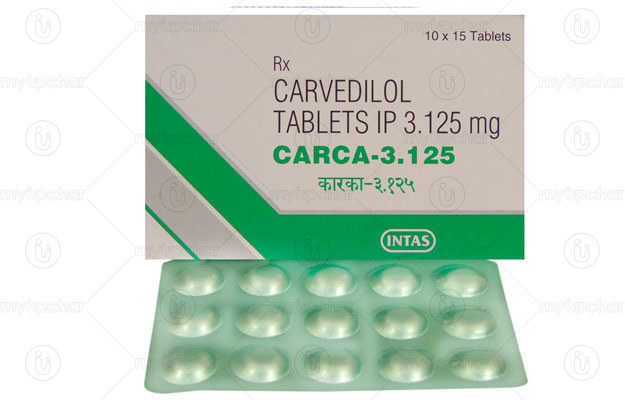
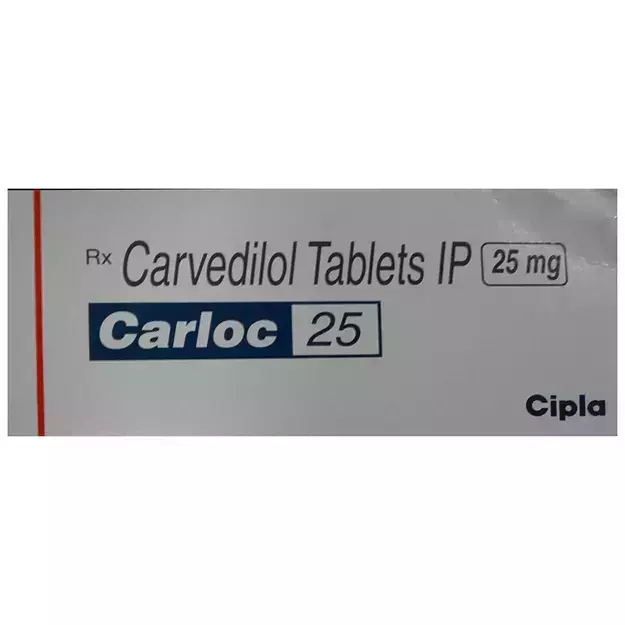
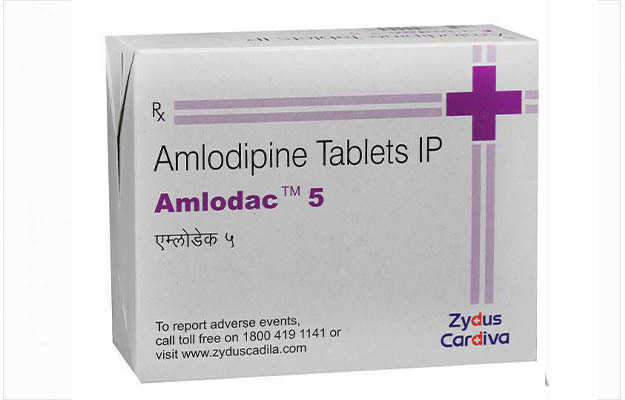
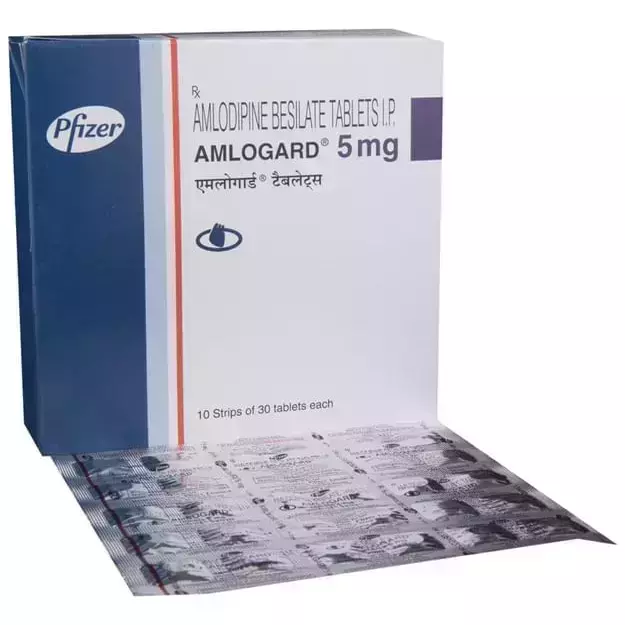
Vicard T 2 Tablet is a commercial drug that is prescribed in the form of Tablet. It is typically used for the treatment of High BP. Vicard T 2 Tablet also has some secondary and off-label uses. These are listed below.
Medical history of the patient along with age and gender determines the dosage of Vicard T 2 Tablet. The condition it has been prescribed for, and the route of administration also determine the right dosage. Refer to the dosage section for a detailed discussion.
The most common side effects of Vicard T 2 Tablet are Nasal congestion, Sinus inflammation. Apart from the aforementioned side effects, Vicard T 2 Tablet can also lead to other problems, which have been listed below. Normally, these side effects of Vicard T 2 Tablet are not long lasting and go away when the treatment is finished. Consult your doctor if these side effects become worse or stay for a longer duration.
Furthermore, you should know that effect of Vicard T 2 Tablet is Severe for pregnant women and Severe for women who are breastfeeding. Further, the section on Vicard T 2 Tablet related warnings talks about Vicard T 2 Tablet's effects on the liver, heart and kidney.
Vicard T 2 Tablet is contraindicated in people with pre-existing medical conditions like Low Blood Pressure (Hypotension) as it can result in adverse effects. The section on Vicard T 2 Tablet contraindications lists all such conditions.
Additionally, Vicard T 2 Tablet may also adversely react with other medicines. See below for a complete list.
In addition to these precautions, you may also note that Vicard T 2 Tablet is not safe while driving, and is is not addictive in nature.
X