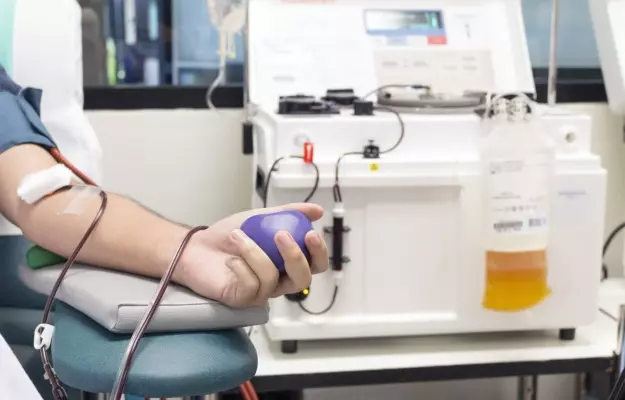
Earlier today (2 July 2020), Delhi chief minister Arvind Kejriwal inaugurated India’s first blood plasma bank for COVID-19 at the Institute of Liver and Biliary Sciences or ILBS hospital. The move comes in the midst of a global pandemic. The hope is that more and more patients who have recovered from COVID-19 will come forward to donate plasma.
Plasma is the yellowish liquid that’s left over after you remove red blood cells, white blood cells and platelets from blood. In short, it is the thing that makes blood liquid. Plasma contains mostly water, and some blood clotting factors (fibrinogens), electrolytes, proteins like albumin and immunoglobulins (better known as antibodies).
Of late, there has been a lot of interest in blood plasma. The reason: plasma from people who have recovered from COVID-19 may be used in an experimental therapy to treat moderately ill patients who are not responding to any treatment, including steroids like methylprednisolone and dexamethasone.
Here’s how it works: People who recover from COVID-19 do so when their body is able to produce enough antibodies against the infection. These antibodies are transported throughout the body in their blood plasma.
Now, antibodies are usually specific to a pathogen. Let’s stick with the example of viruses. So an antibody for Nipah virus would be different from an antibody for Ebola disease, swine flu, dengue or COVID-19 and so on.
When you take antibodies from a recently recovered patient and put them in someone else, the antibodies help patient 2 fight off the disease (and the resulting inflammation) for a short time. Ideally, giving patient 2’s body enough time to form its own antibodies and defences against the infection.
This kind of therapy is known as convalescent plasma therapy or passive antibody therapy. It is far from new. In fact, its antecedents go back over 100 years: it was used in the treatment of viral infections like polio, measles and mumps before a vaccine was developed for them.
Doctors became interested in the therapy for COVID-19 in March after medical practitioners in China tried it successfully in the treatment of five critically ill COVID-19 patients who had developed acute respiratory distress syndrome.
In April, India’s apex medical research body Indian Council of Medical Research started clinical trials for COVID-19 convalescent plasma therapy for moderately ill patients.
As it turns out, this was a timely step: since then, the number of COVID-19 cases has gone up more than 2400% to cross six lakh at the beginning of July. The number of people who have recovered from the illness has crossed 3.5 lakh already.
So the question now arises, who among the 3.5 lakh (and counting) can actually donate plasma, for the trial and experimental treatment of other patients who fall sick after them?
Who can donate blood plasma for COVID-19 treatment?
COVID-19 is a new viral infection, and we don’t yet have an official treatment or vaccine for it. Given this, convalescent plasma therapy has received emergency approvals in some countries—in India, it is considered an experimental therapy. The Indian Council of Medical Research (ICMR) began a multi-centre trial of this therapy on 22 April 2020. According to the protocols set for the trial, potential blood plasma donors should meet the following criteria:
• Be over 18 years of age
• Weigh over 55 kilograms
Recovered female patients who do not have children can donate (more on this later)
• Recovered patients should have had the RT-PCR for COVID-19 and at least two symptoms: fever and cough
• Patients should have recovered completely 28 days before donation. They can also donate plasma 14 days after recovery, provided they test negative twice on the RT-PCR test for COVID-19—the samples must be taken with a nasopharyngeal swab and collected 24 hours apart.
Most successful donors can donate convalescent plasma again after two weeks.
In addition to these, every donor must:
-
Undergo physical examination
-
Be deemed fit to donate blood plasma, based on their medical history
-
Not have had transfusion of blood products for at least eight weeks prior to donation
-
Undergo testing for
-
Blood group
-
Clinically significant antibodies (extended Rh, Kell, Duffy, Kidd, MNS): you may not be allowed to donate plasma if you test positive for these antibodies.
-
Complete blood count, including haemoglobin and platelet count. You will only be allowed to donate plasma if your haemoglobin is over 12.5g/dl and your platelet count exceeds 1.5 lakh platelets per microliter of blood.
-
Test negative for HIV, hepatitis B, hepatitis C, syphilis and malaria
-
Have total serum protein more than 6gm/dl, as per Drugs and Cosmetics (Second Amendment) Rules, 2020
-
Undergo testing for IgG and IgM antibodies to COVID-19. “Donors negative for IgG or positive for IgM antibodies to COVID 19 will be deferred,” according to the ICMR protocol. This may be because IgM antibodies usually signal active infection.
-
Where possible, ICMR has recommended the titration (quantitative analysis) of SARS-CoV-2 neutralizing antibodies—both IgG and IgM—be done on site to ensure that the blood plasma has adequate antibodies before it is transfused into a patient who needs urgent help. Where this is not possible on site, there are protocols to send samples to the National Institute of Virology in Pune for testing.
Can women donate blood plasma?
Females can donate blood plasma provided they meet the requirements: they must be over 18 years old, weigh more than 55 kilograms, have recovered from COVID-19 recently and not have children (nulliparous).
This is because women who have been pregnant are more likely to have something called HLA antibodies that can produce adverse events like transfusion-related acute lung
injury or TRALI in the recipient of the plasma. TRALI can, in turn, lead to fluid build-up in the lungs or pulmonary edema.
The added requirement for female donors is not limited to blood plasma donation for COVID-19. In some countries, moms and women who have ever been pregnant are now being screened for HLA antibodies before any kind of donation of blood products (platelets included).
HLA stands for human leukocyte antigen.
How is blood plasma taken?
The plasma is taken by a process known as plasmapheresis.
-
If you offer to donate blood after recovering from COVID-19, a doctor will first explain the procedure and possible adverse events to you. (Possible adverse effects of donating blood plasma include hypotensive reactions (drop in blood pressure), citrate reactions (tingling feeling), hematomas, loss of consciousness, seizures and allergy. Typically, they resolve quickly once the procedure is stopped.)
-
The doctor will then conduct the examination and tests. If you meet all the test-based criteria, your doctor will give you a choice of dates to come back for the plasma donation.
-
For the plasma collection, a medical practitioner will hook you up to a machine. Your blood will go into the machine where plasma will be separated from everything else by centrifugal separation (a kind of spinning action). The actual procedure takes about 1 hour.
-
Rest assured that there are protocols in place to ensure the donor’s safety. For example, the volume of plasma collected can not exceed 500 ml per sitting according to the Drugs and Cosmetics (Second Amendment) Rules, 2020). Also, at all points in the procedure, the volume of blood outside the body (in the machine) is less than 15% of your total blood volume.
-
The collected plasma is stored in 200 ml packs and frozen within 8 hours. It is then stored at less than minus-40 degree Celsius until it is needed for transfusion—at which point, it is warmed up to 37 degrees Celsius.
If you are in Delhi and wish to donate plasma, you can call 1031 or send a WhatsApp on 8800007722 to register.
References
https://www.rrvbc.org/wp-content/uploads/2014/10/RRVBC_HLA_TechnicalNotificationSheet_w-Bleeds_PRF.pdf Rock River Valley Blood Centre, US. Important Information for Female Platelet Donors
https://jamanetwork.com/journals/jama/fullarticle/2763983 Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma
Chenguang Shen, Zhaoqin Wang, Fang Zhao, et al. [link]. JAMA, 27 March 2020; 323(16): 1582-1589.
https://www.redcrossblood.org/faq.html#donating-blood-covid-19-convalescent-plasma COVID-19 Convalescent plasma. The American National Red Cross
https://www.ncbi.nlm.nih.gov/books/NBK531504/ Mathew J, Sankar P, Varacallo M. Physiology, Blood Plasma. [Updated 2020 Apr 25]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK531504/
https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/donate-covid-19-plasma Donate COVID-19 Plasma US Food & Drug Administration
https://www.nhsbt.nhs.uk/covid-19-research/plasma-donors/who-can-donate-plasma/ NHS Blood and Transplant, UK. COVID-19 research: who can donate plasma?
https://cdsco.gov.in/opencms/resources/UploadCDSCOWeb/2018/UploadPublic_NoticesFiles/amended%20Clinical%20Trial%20protocol%20version%201.4%20%20by%20ICMR%20dated%202020.pdf A Phase II, Open Label, Randomized Controlled Trial to Assess the Safety and Efficacy of Convalescent Plasma to Limit COVID-19 Associated Complications in Moderate Disease. Indian Council of Medical Research, Convalescent Plasma in COVID-19: Version 1.4 Dated 22nd April, 2020