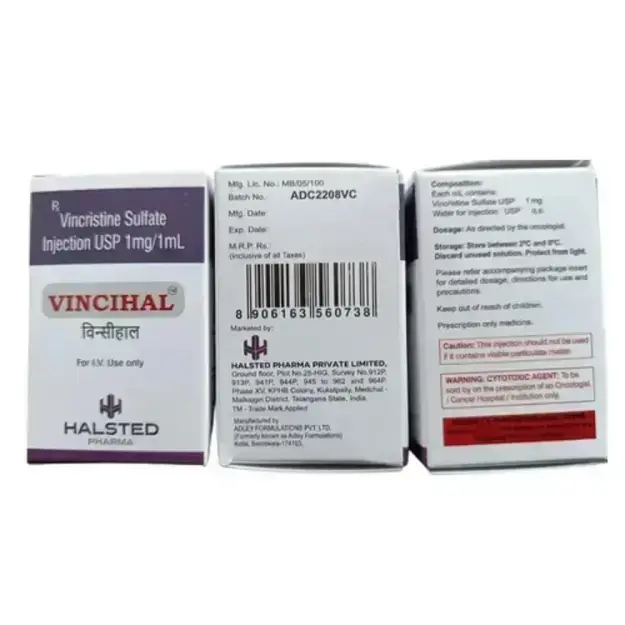
Vincihal is consists of Vincristine is a chemotherapy medication derived from the Madagascar periwinkle plant (Catharanthus roseus). It belongs to the vinca alkaloid class and is widely used in the treatment of various cancers, including leukemias, lymphomas, solid tumors, and pediatric malignancies. Vincristine exerts its antineoplastic effects by inhibiting microtubule formation, thereby preventing cancer cell division. It is a cornerstone in combination chemotherapy regimens such as CHOP (for Non-Hodgkin’s Lymphoma) and ALL treatment protocols (for Acute Lymphoblastic Leukemia). Despite its high therapeutic efficacy, Vincristine is associated with dose-limiting neurotoxicity, particularly peripheral neuropathy. Careful dose adjustments and monitoring are essential for optimizing its benefits while minimizing adverse effects.
Mechanism of Action
Vincristine is a mitotic inhibitor that binds to tubulin, a key protein responsible for microtubule assembly. By preventing microtubule polymerization, Vincristine disrupts mitotic spindle formation, leading to cell cycle arrest at the metaphase and subsequent apoptosis in rapidly dividing cancer cells.
Key Mechanisms:
Microtubule Inhibition – Blocks mitotic spindle formation, preventing cell division.
Apoptosis Induction – Triggers programmed cell death in rapidly dividing cancer cells.
Inhibition of Angiogenesis – Reduces the formation of new blood vessels that support tumor growth.
Indications and Uses
1. Hematologic Malignancies (Blood Cancers)
Acute Lymphoblastic Leukemia (ALL) – Used in induction, consolidation, and maintenance phases.
Non-Hodgkin’s Lymphoma (NHL) – Part of CHOP regimen (Cyclophosphamide, Doxorubicin, Vincristine, Prednisone).
Hodgkin’s Lymphoma (HL) – Component of ABVD (Adriamycin, Bleomycin, Vinblastine, Dacarbazine) regimen.
Multiple Myeloma (MM) – Used in combination therapies.
2. Pediatric Cancers
Wilms' Tumor
Neuroblastoma
Rhabdomyosarcoma
Ewing’s Sarcoma
3. Solid Tumors
Small Cell Lung Cancer (SCLC)
Breast Cancer
Ovarian Cancer
4. Other Indications
Immune Thrombocytopenic Purpura (ITP) – Occasionally used in refractory cases.
Vincristine remains one of the most effective chemotherapy agents for hematologic and pediatric cancers. It has significantly improved survival rates for leukemia, lymphoma, and neuroblastoma patients. However, neurotoxicity remains a major limitation, requiring careful dosing and monitoring. With ongoing research into liposomal formulations and targeted drug delivery, the future of Vincristine in oncology continues to evolve. Always administer intravenously, NEVER intrathecally, Monitor for neuropathy, constipation, and drug interactions and dose capping at 2 mg reduces toxicity risks.
Dosage and Administration
Vincristine is administered via intravenous (IV) injection.
| Indication |
Typical Dose |
Frequency |
| ALL (Induction) |
1.4 mg/m² IV |
Once weekly |
| NHL (CHOP Regimen) |
1.4 mg/m² IV |
Day 1 of each cycle |
| HL (ABVD Regimen) |
1.4 mg/m² IV |
Every 2 weeks |
| Wilms’ Tumor, Neuroblastoma |
1.5 mg/m² IV |
Weekly |
| Maximum Dose |
2 mg per dose (to limit neurotoxicity) |
|
Pediatric Dosing is adjusted based on Body Surface Area (BSA).
Dose Capping at 2 mg per administration is recommended to reduce neurotoxicity risk.
Administration Warning: Vincristine is NEVER given intrathecally (into the spinal cord) as it can cause fatal neurotoxicity.
Efficacy and Clinical Studies
1. Acute Lymphoblastic Leukemia (ALL)
Studies show 80–90% remission rates when Vincristine is combined with corticosteroids and asparaginase.
Maintenance therapy with Vincristine + Methotrexate + Mercaptopurine improves survival.
2. Lymphomas (Hodgkin’s and Non-Hodgkin’s)
CHOP and ABVD regimens show high remission rates (~70-80%).
Survival benefits when combined with radiation therapy in advanced stages.
3. Pediatric Tumors
Neuroblastoma and Wilms' tumor respond well to Vincristine + Cyclophosphamide + Doxorubicin combinations.
Side Effects and Toxicity
Common Side Effects:
Peripheral Neuropathy – Tingling, numbness, foot drop, wrist drop.
Gastrointestinal Issues – Constipation, nausea, vomiting.
Alopecia (Hair Loss) – Temporary but significant.
Fatigue – Generalized weakness.
Serious Side Effects:
Severe Neurotoxicity – Dose-limiting, may cause paralytic ileus, vocal cord paralysis, or motor dysfunction.
Myelosuppression – Less severe than other chemotherapy agents but still requires monitoring.
SIADH (Syndrome of Inappropriate ADH Secretion) – Can cause hyponatremia (low sodium levels).
Tumor Lysis Syndrome (TLS) – Can occur in aggressive leukemias and lymphomas.
Fatal Toxicity:
Intrathecal Administration – Always contraindicated; causes irreversible CNS damage and death.
Contraindications and Precautions
Contraindications:
Severe neuropathy.
Hypersensitivity to vinca alkaloids.
Pregnancy & Breastfeeding – Teratogenic effects reported.
Precautions:
Neuropathy Monitoring: Reduce dose if severe neuropathy occurs.
Liver Dysfunction: Dose adjustments needed.
Gastrointestinal Paralysis: Monitor for constipation or paralytic ileus.
Drug Interactions
Vincristine is metabolized by the CYP3A4 enzyme, leading to multiple drug interactions.
CYP3A4 Inhibitors (Ketoconazole, Erythromycin, Fluconazole) – Increase Vincristine toxicity.
CYP3A4 Inducers (Rifampin, Carbamazepine, Phenytoin) – Reduce Vincristine efficacy.
Azole Antifungals (Itraconazole, Voriconazole) – Exacerbate neurotoxicity.
Other Chemotherapy Drugs (Cisplatin, Doxorubicin) – Increase myelosuppression risk.
Storage and Handling
Storage Conditions:
Keep at 2-8°C (Refrigerated).
Protect from light exposure.
Avoid freezing.
Safe Handling:
Vincristine is a cytotoxic drug. Wear gloves and protective clothing when handling.
Proper Disposal: Dispose of per local hazardous waste guidelines.
X