Lupin Ltd on 23 July announced that it had received tentative approval from the United States Food and Drug Administration (US FDA)—the top government drug agency in the US—to sell type 2 diabetes medicines empagliflozin and linagliptin in that country.
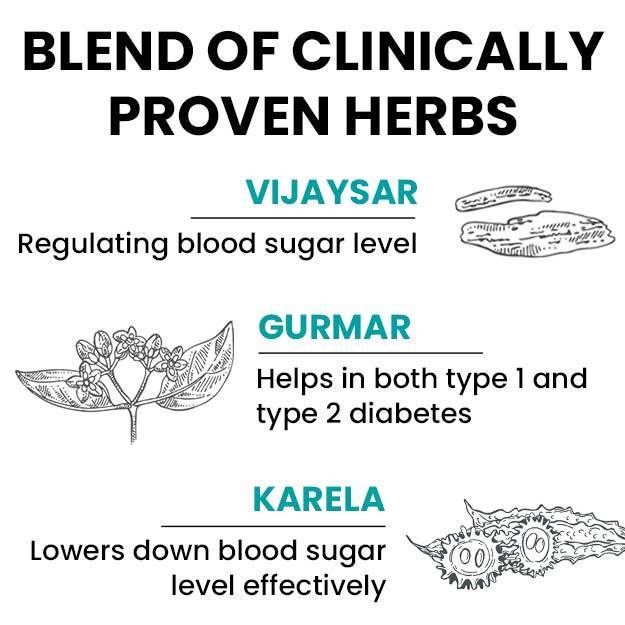
Data show that roughly 10% of the American population lives with type 2 diabetes—a metabolic disorder in which the body is unable to properly utilize blood glucose for energy. Needless to say, lifestyle-linked diabetes is a major health concern in the US (as it is in India).
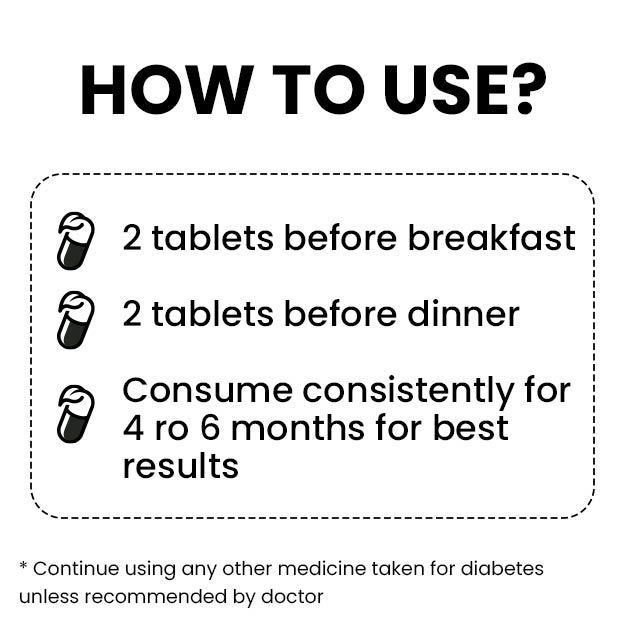
Empagliflozin and linagliptin are generic versions of Glyxambi®, developed by the German pharmaceutical company Boehringer Ingelheim Pharmaceuticals, Inc. These tablets are used jointly with diet control and exercise to improve blood sugar levels in adults with type 2 diabetes.
After receiving approval from the FDA, Lupin issued a statement saying that the company had been given experimental approval for these drugs. Under tentative approval, the FDA has allowed Lupin to sell these drugs with the strengths of 10 mg / 5 mg and 25 mg / 5 mg.